Molecular Graphics and Visualization
The visualization of field lines of the membrane-inserted BLT2 GPCR is implemented as WebGL-page using SpiderGl. This animation shown on a seperate webpage is supplementary material to the article Electrostatically-driven fast association and perdeuteration allow transferred cross-relaxation detection for G protein-coupled receptor ligands with equilibrium dissociation constants in the high-to-low nanomolar range by Catoire et al., Journal of Biomolecular NMR 2011.
Such molecular visualization applications of SpiderGL are described in more detail in M. Callieri et al., Visualization methods for molecular studies on the web platform, The Web3D 2010 Conference, 22-24 July 2010, Los Angeles, California
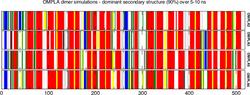
The above figure shows barcharts representing the dominant secondary structure of a protein calculated from the secondary structure time series for a given molecular dynamics simulation. It shows secondary structure elements that are present for at least 90% of the simulation time for the various simulation runs. The dominant secondary structure analysis makes secondary structure comparisons between different simulations easier. This representation was introduced in the recently published computational work on OmpT where we describe molecular dynamics simulations and docking of several tetra-peptides [[Baaden 2004]] and has since been used in a number of computational studies of membrane proteins. The graphs were produced via a script run over the production data of all simulations. The script was specifically developed for this purpose and uses SDF, GNU make, Gromacs, dssp, perl, imagemagick and xmgrace.